Consultancy Service
Dr. Bhaskar's Process Solutions offers a comprehensive suite of services designed to meet to the diverse needs of chemical and pharmaceutical industry. Our commitment to excellence ensures that our customers receive services that are both innovative and reliable.
Process Research & Development

We stand ready to assist and collaborate with your R&D team to support process research and development for APIs/ Intermediartes and related molecules. We support on to develop a novel process or improvement of an existing process. Key services include:
- Assessment of quality target product profile (QTPP) and critical quality attributes (CQAs) of an API / Intermediate.
- Route of synthesis (ROS) design for a proposed API/ Intermediate.
- Feasibility studies, evaluation of rawmaterils cost (RMC) and justification for selected ROS.
- Intellectual Property (IP) management aspects such as patent infringement analysis, design of patent non-infringing process.
- Process optimization based on quality by design (QbD) approach and Process validation.
- Supporting on experiment design to improve process efficiency and cost reduction.
Process Scale-up and Tech Transfer
To achieve success in process scale-up and technology transfer collaborative effort between different departments including R&D, quality control, quality assurance, production and environment health and safety (EHS) teams is required. Key services include:
- Support documentation to ensure recording of critical information, provide guidance for the preparation of technology transfer documents (PDR/TTD).
- Supporting on review of third-party technology transfer documents and to help on process scale-up and technology transfer to manufacturing site.
- Assessment and control of impurities (e.g. process, degradation, genotoxic, nitrosamine and elemental impurities) to ensure product quality
- Process review with a science-based quality risk management (QRM) approach to ensure product quality and safety.
- Perform gap analysis and suggest required control experiments (e.g., what if studies) to achieve “First Time Right” and to ensure process robustness.
- To help on troubleshooting a chemical process during scale-up and manufacture.
Structural Elucidation & Characterization

Structural elucidation and characterization is an important aspect to ensure the quality of raw materials, intermediates and final API and reference standards used for analytical method development. Key services include:
- Helping on use of right analytical techniques for structural elucidation and characterization.
- Analytical services (e.g. NMR, Mass, LC-MS, IR, UV/VIS, DSC, TGA and XRD)
- Interpretation of analytical data for characterization of APIs, Intermediates, KSMs and impurities;
- identification and structure elucidation of known and/ unknown impurities;
- characterization of different polymorphic forms of an API;
- To support on preparation of structural confirmation report.
Solid State Chemistry

Polymorphism is the property of a solid-state chemical substance to exist in different crystalline forms. The subject of polymorphism has a special interest in pharmaceutical industry as it affects discovery, development and manufacturing process of a drug substance and has impact in legal and regulatory decisions. Key services include:
- Polymorph screening of APIs
- Different solid forms of an API – salts, co-crystals, solvates and hydrates.
- Amorphous solids
- Characterization of solid forms
- Intellectual property and Regulatory issues
Contained Chemistry

Leveraging the continual understanding of disease with advanced technologies showed a significant and increasing portion of high potent drugs in pharmaceutical industry. Key area of services offered in potent API process development and manufacture include:
- Categorization of potent compounds.
- Process development
- Facility design and containment controls for safe handling of potent compounds and manufacture operations.
- Cleaning and decontamination procedures.
- Training
Complex APIs and Intermediates
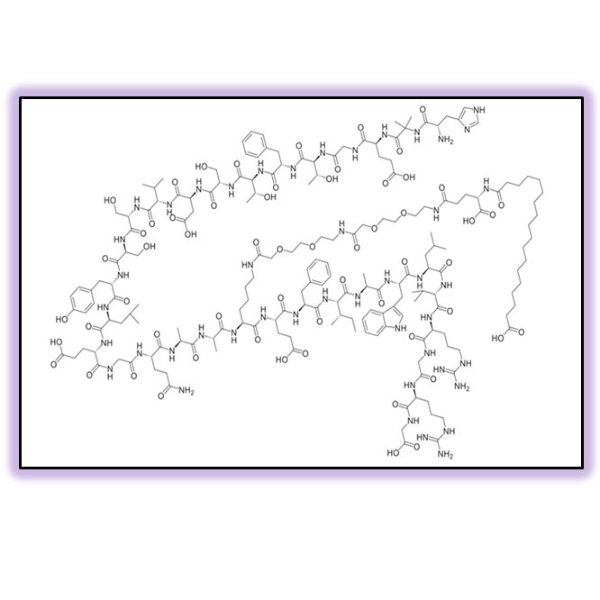
A complex API refers to a chemical substance or a mixture of substances with intricate molecular structures used as an active therapeutic component in a medication. Examples of complex APIs include: small molecule with many chiral atoms (e.g., Eribulin), cross-linked polymers (e.g. Colesevelam HCl), semi-synthetic mixtures (e.g. Enoxaparin), synthetic peptides (e.g. Linaclotide) and oligonucleotides (e.g. Nusinersen). We support on
- process development of complex APIs and Intermediates
- process controls such as starting material specifications and in-process and intermediate controls to ensure quality of final API and to obtain consistent yield.
- Structural elucidation and characterization
- Documentation of CMC portion for DMF Regulatory Submission.

